The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
1.1. Description of the Intervention
1.2. How This Intervention Might Work
1.3. Why It Is Important to Do This Review
1.4. Aim
2. Methods
2.1. Types of Studies
2.2. Types of Participants
2.3. Types of Interventions
2.4. The Inclusion Criteria
2.5. The Exclusion Criteria
2.6. Types of Outcome Measures
- Gut microbiota;
- Blood glucose parameters: glycated haemoglobin (HbA1c, %);
- Inflammatory markers: tumour necrosis factor α (TNF-α); high-sensitivity C-reactive protein (hsCRP);
- Body mass index (BMI) (Kg/m2).
- Secondary outcome measures of interest:
- Fasting blood glucose (FBG, mmol/L);
- Postprandial blood glucose (2 h PBG, mmol/L);
- Homeostatic model assessment of insulin resistance (HOMA–IR);
- Glucagon-like peptide-1 (GLP-1);
- Fasting insulin.
2.7. Search Methods for Identification of Studies
3. Data Collection and Analysis
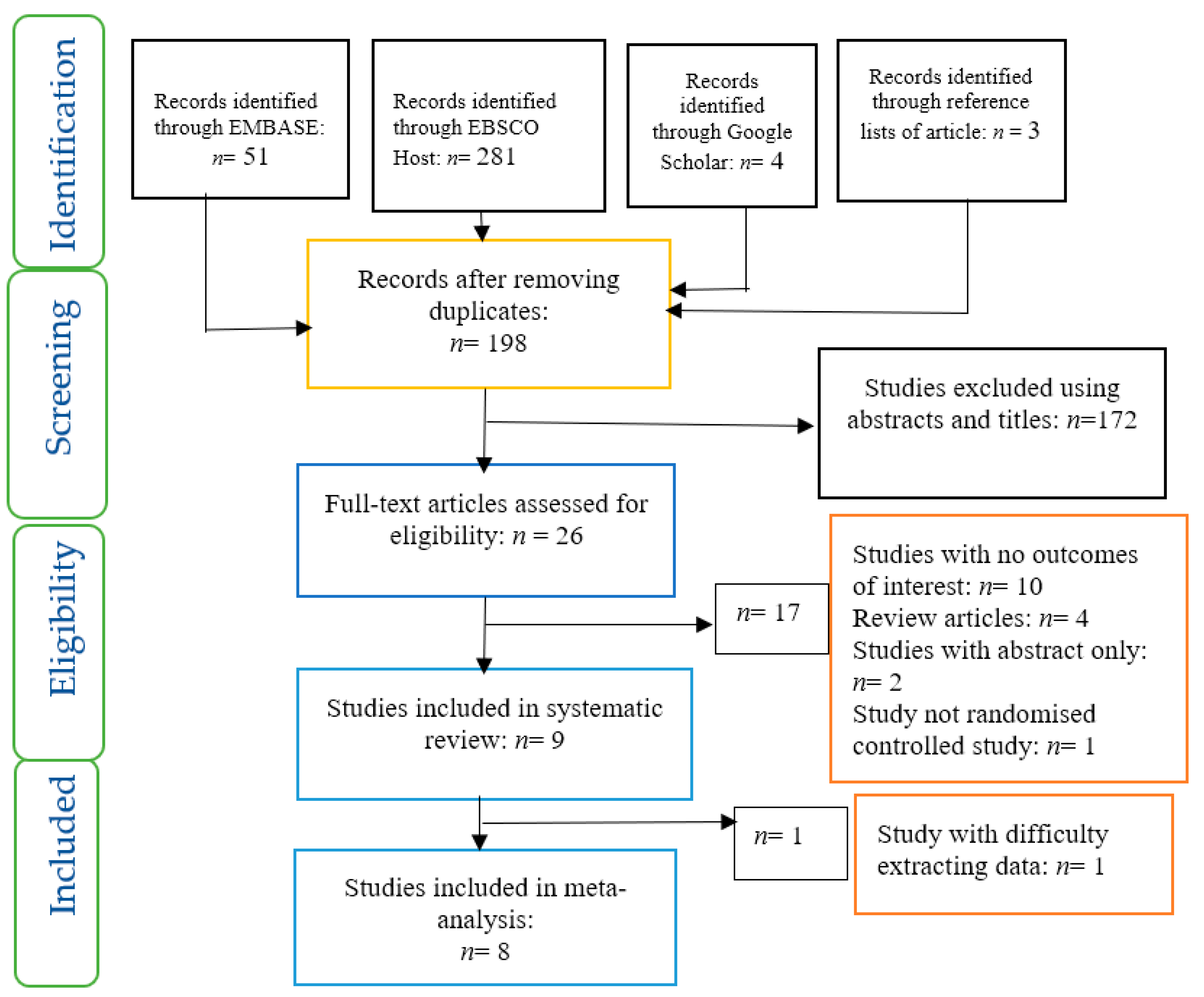
3.1. Selection of Studies
3.2. Data Extraction and Management
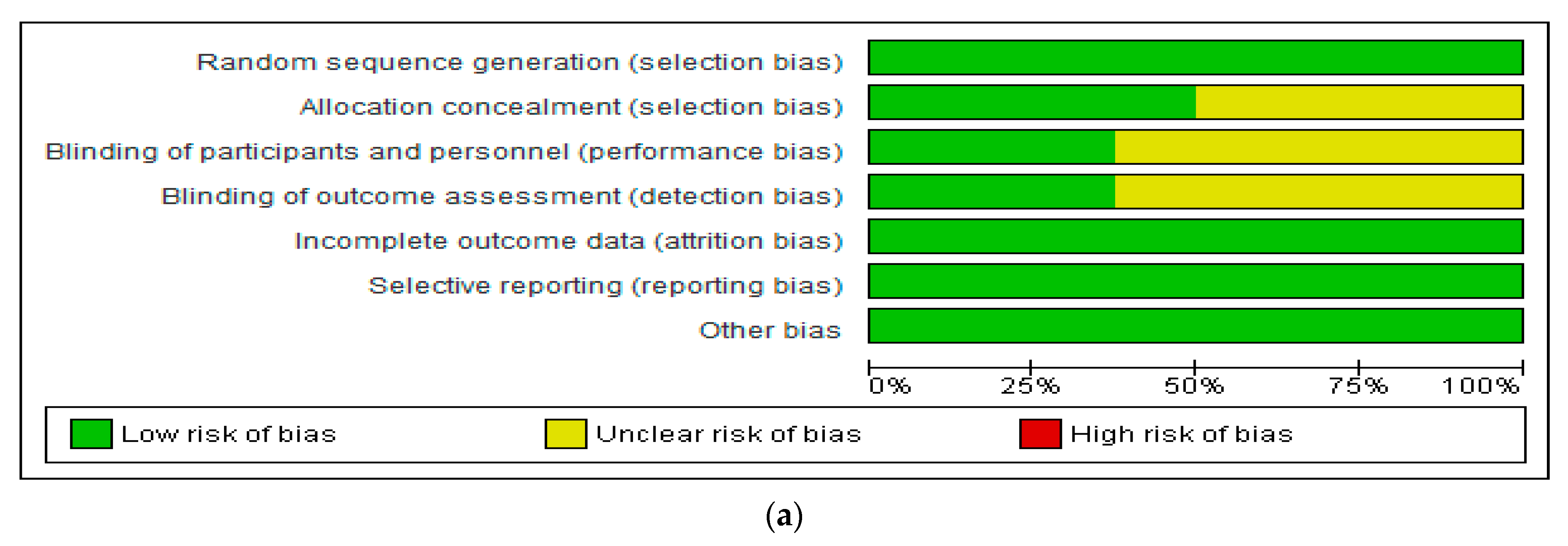
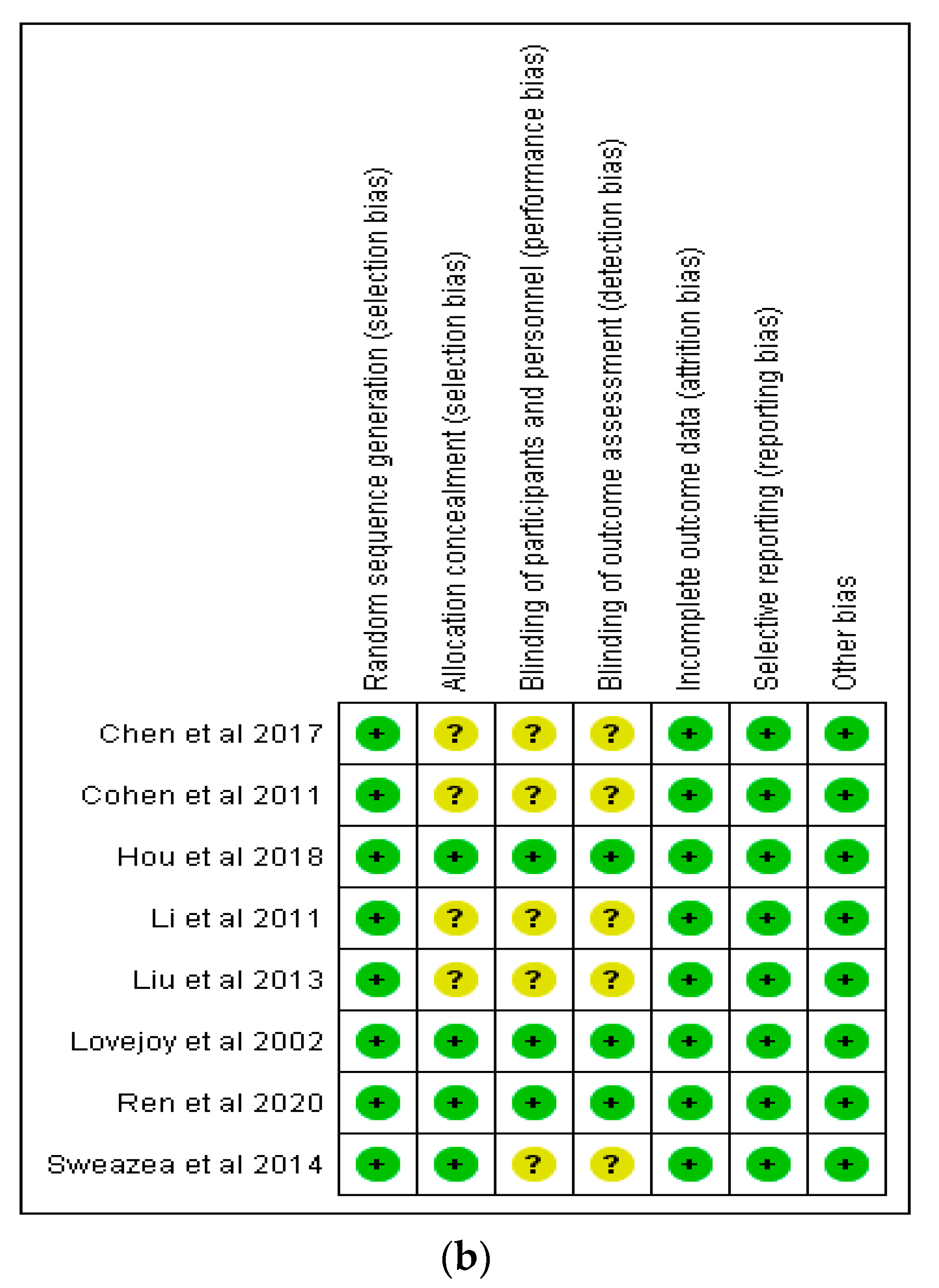
3.3. Assessment of Risk of Bias in Included Studies
3.4. Data Analysis
4. Results
4.1. Evaluation of the Risk of Bias of Included Studies
4.2. Gut Microbiota
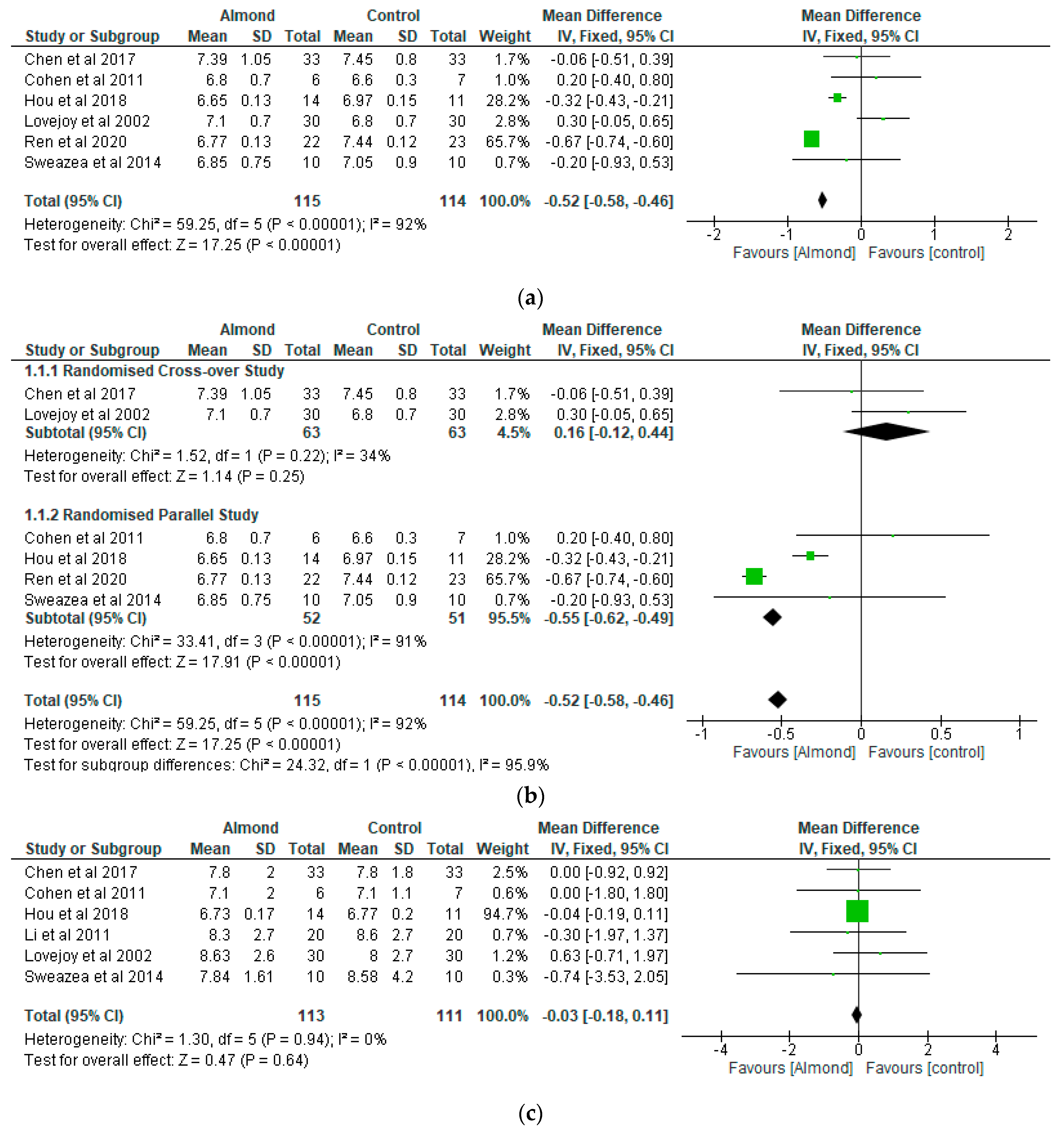
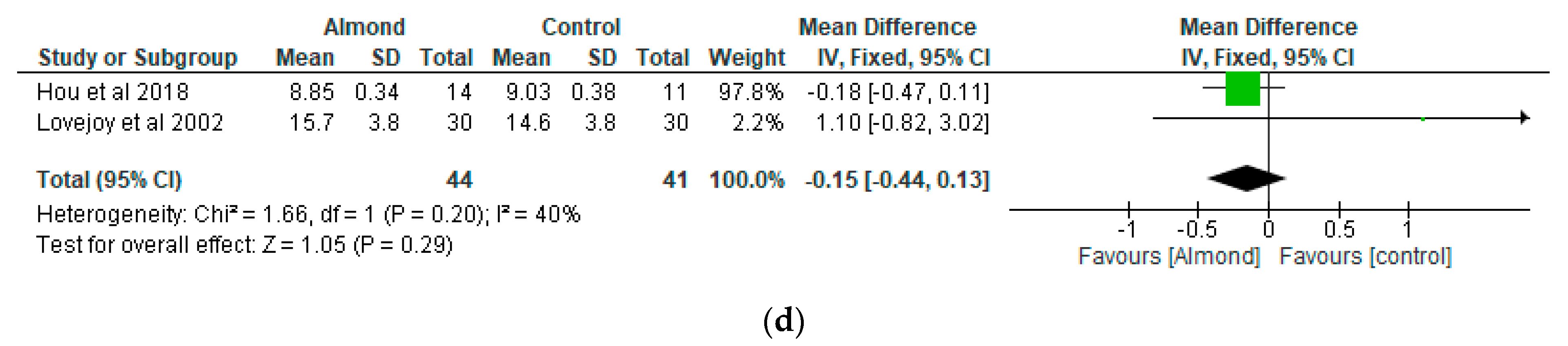
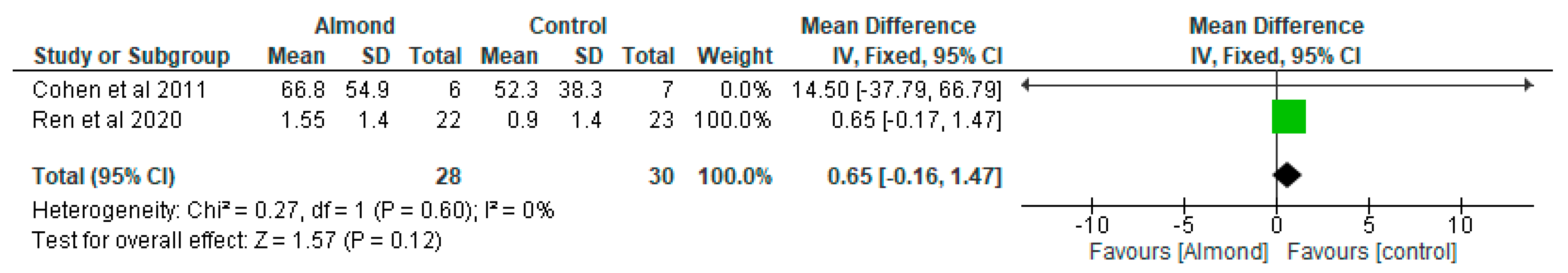
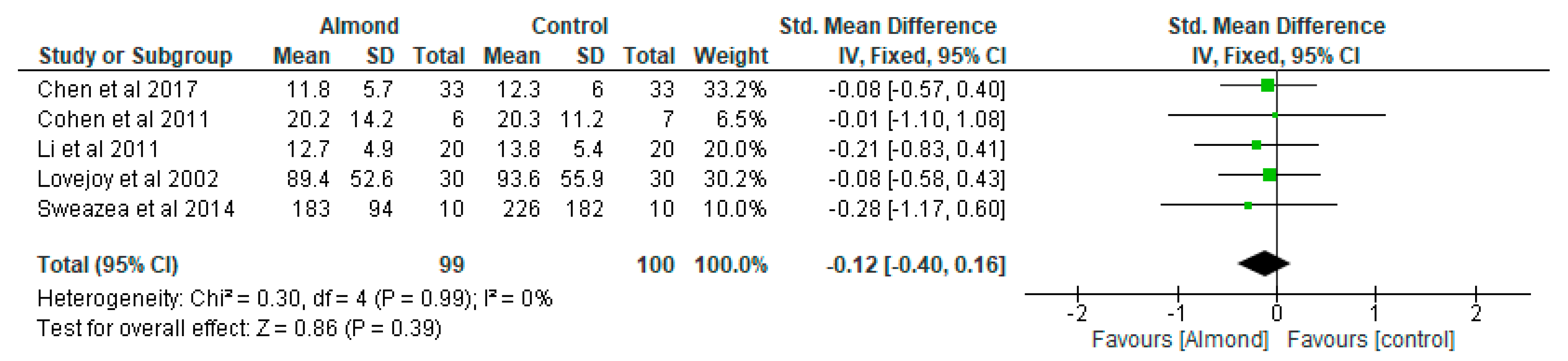
4.3. Glycaemic Control
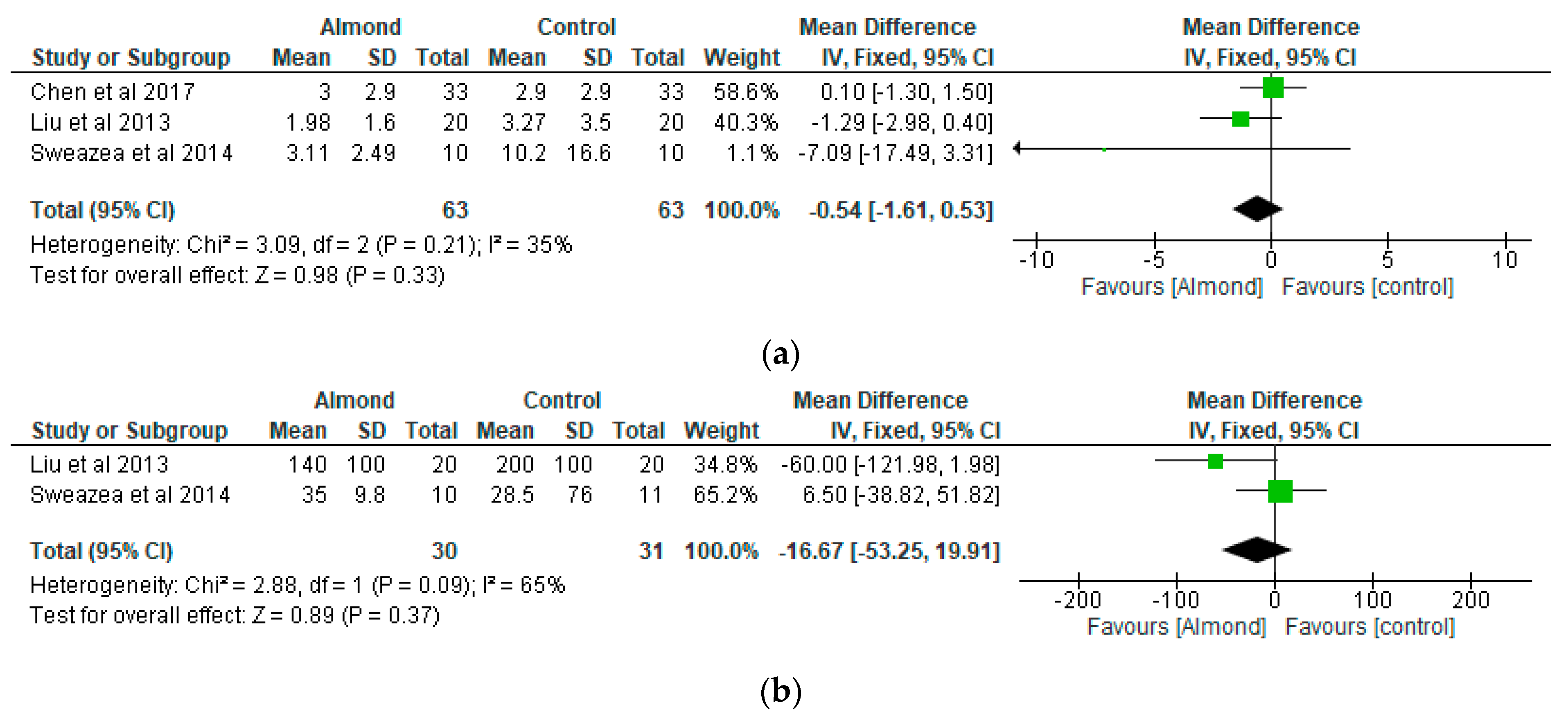
4.4. Inflammatory Markers
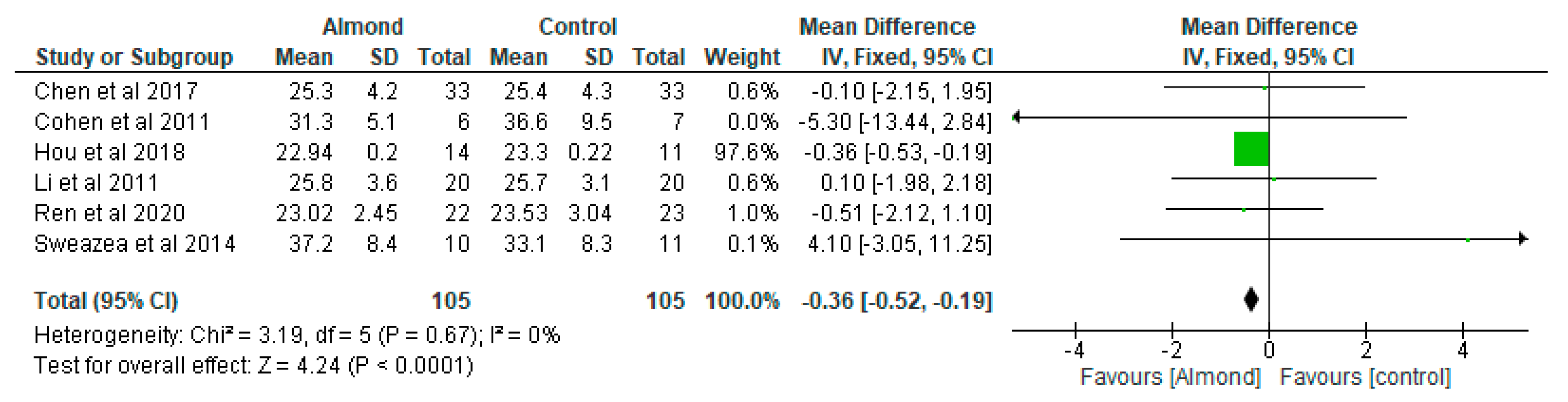
4.5. Body Mass Index (BMI) (Kg/m2)
4.6. Homeostatic Model Assessment of Insulin Resistance (HOMA–IR)
4.7. Glucagon-Like Peptide-1 (GLP-1)
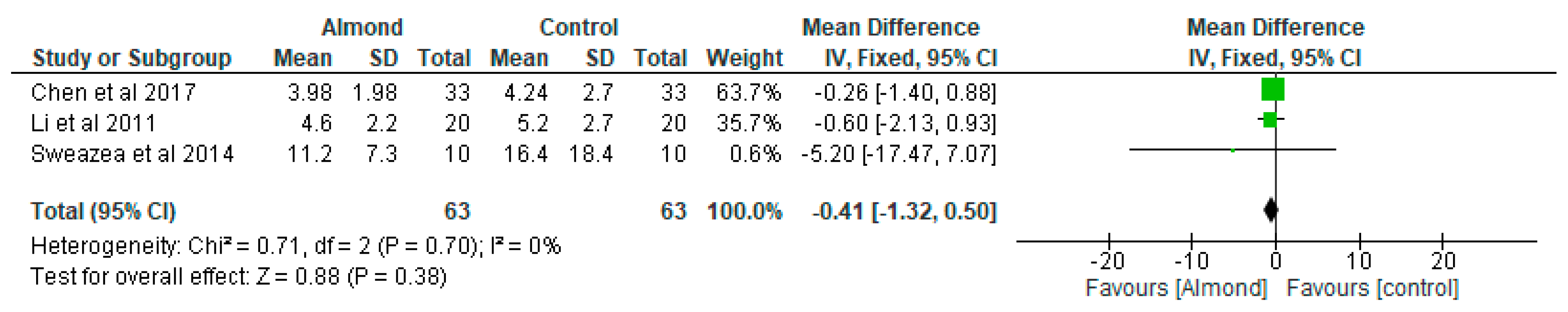
4.8. Fasting Insulin
5. Discussion
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. Promoting Diabetes Care, Prevention and a Cure Worldwide. 2019. Available online: https://sites.pitt.edu/~super1/Metabolic/IDF5.pdf (accessed on 23 August 2021).
- National Collaborating Centre for Chronic Conditions (NCCCC). Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update); Royal College of Physicians: London, UK, 2008. [Google Scholar]
- Bagust, A.; Hopkinson, P.K.; Maslove, L.; Currie, C.J. The projected health care burden of Type 2 diabetes in the UK from 2000 to 2060. Diabet. Med. 2002, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Diabetes UK (2012) NHS Spending on Diabetes ‘to Reach £16.9 Billion by 2035’. Available online: https://www.diabetes.org.uk/about_us/news_landing_page/nhs-spending-on-diabetes-to-reach-169-billion-by-2035 (accessed on 29 June 2021).
- Mori, A.M.; Considine, R.V.; Mattes, R.D. Acute and second-meal effects of almond form in impaired glucose tolerant adults: A randomized crossover trial. Nutr. Metab. 2011, 8, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.-Y.; Ojo, O.; Wang, L.-L.; Wang, Q.; Jiang, Q.; Shao, X.-Y.; Wang, X.-H. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Kendall, C.W.C.; Lamarche, B.; Banach, M.S.; Srichaikul, K.; Vidgen, E.; Mitchell, S.; Parker, T.; Nishi, S.; Bashyam, B.; et al. Nuts as a replacement for carbohydrates in the diabetic diet: A reanalysis of a randomised controlled trial. Diabetologia 2018, 61, 1734–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds ( Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Zand, N.; Wang, X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 1805. [Google Scholar] [CrossRef]
- Reimer, R.A.; Wharton, S.; Green, T.J.; Manjoo, P.; Ramay, H.R.; Lyon, M.R.; Gahler, R.J.; Wood, S. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur. J. Nutr. 2021, 60, 1237–1251. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- King, J.C.; Blumberg, J.; Ingwersen, L.; Jenab, M.; Tucker, K.L. Tree nuts and peanuts as components of a healthy diet. J. Nutr. 2008, 138, 1736S–1740S. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef]
- Wien, M.A.; Sabate, J.M.; Ikle, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, S.; Misra, A.; Pandey, R. Effect of Almond Supplementation on Glycemia and Cardiovascular Risk Factors in Asian Indians in North India with Type 2 Diabetes Mellitus: A 24–Week Study. Metab. Syndr. Relat. Disord. 2017, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Blanco Mejia, S.; Kendall, C.W.C.; Viguiliouk, E.; Augustin, L.S.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Maroleanu, A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of tree nuts on metabolic syndrome criteria: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2014, 4, e004660. [Google Scholar] [CrossRef] [PubMed]
- Muley, A.; Fernandez, R.; Ellwood, L.; Muley, P.; Shah, M. Effect of tree nuts on glycemic outcomes in adults with type 2 diabetes mellitus: A systematic review. JBI Evid. Synth. 2021, 19, 966–1002. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Kendall, C.W.C.; Blanco Mejia, S.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Jayalath, V.H.; Augustin, L.S.A.; Chiavaroli, L.; Leiter, L.A.; et al. Effect of Tree Nuts on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Dietary Trials. PLoS ONE 2014, 9, e0103376. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Salehi-Abargouei, A.; Salas-Salvadó, J.; Guasch-Ferré, M.; Humphries, K.; Sarrafzadegan, N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Am. J. Clin. Nutr. 2015, 101, 966–982. [Google Scholar] [CrossRef] [Green Version]
- Musa-Veloso, K.; Paulionis, L.; Poon, T.; Lee, H.Y. The effects of almond consumption on fasting blood lipid levels: A systematic review and meta-analysis of randomised controlled trials. J. Nutr. Sci. 2016, 5, e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- The Nordic Cochrane Centre. Review Manager, Version 5.3; The Nordic Cochrane Centre; The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bodnaruc, A.M.; Prud’homme, D.; Giroux, I. Acute effects of an isocaloric macronutrient-matched breakfast meal containing almonds on glycemic, hormonal, and appetite responses in men with type 2 diabetes: A randomized crossover study. Appl. Physiol. Nutr. Metab. 2020, 45, 520–529. [Google Scholar] [CrossRef]
- Chen, C.-M.; Liu, J.-F.; Li, S.-C.; Huang, C.-L.; Hsirh, A.-T.; Weng, S.-F.; Chang, M.-L.; Li, H.-T.; Mohn, E.; Oliver Chen, C.-Y. Almonds ameliorate glycemic control in Chinese patients with better controlled type 2 diabetes: A randomized, crossover, controlled feeding trial. Nutr. Metab. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metab. Clin. Exp. 2011, 60, 1312–1317. [Google Scholar] [CrossRef]
- Li, S.-C.; Liu, Y.-H.; Liu, J.-F.; Chang, W.-H.; Chen, C.-M.; Chen, C.-Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2011, 60, 474–479. [Google Scholar] [CrossRef]
- Liu, J.-F.; Liu, Y.-H.; Chen, C.-M.; Chang, W.-H.; Chen, C.-Y. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: A randomized crossover controlled feeding trial. Eur. J. Nutr. 2013, 52, 927–935. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Most, M.M.; Lefevre, M.; Greenway, F.L.; Rood, J.C. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Sweazea, K.; Johnston, C.; Ricklefs, K.D.; Petersen, K.N. Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. J. Funct. Foods 2014, 10, 252–259. [Google Scholar] [CrossRef]
- Dreher, M.L. A Comprehensive Review of Almond Clinical Trials on Weight Measures, Metabolic Health Biomarkers and Outcomes, and the Gut Microbiota. Nutrients 2021, 13, 1968. [Google Scholar] [CrossRef]
- Mohan, V.; Anjana, R.M.; Gayathri, R.; Ramya Bai, M.; Lakshmipriya, N.; Ruchi, V.; Sudha, V. Glycemic Index of a Novel High-Fiber White Rice Variety Developed in India—A Randomized Control Trial Study. Diabetes Technol. Ther. 2016, 18, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Similä, M.E.; Valsta, L.M.; Kontto, J.P.; Albanes, D.; Virtamo, J. Low-, medium- and high-glycaemic index carbohydrates and risk of type 2 diabetes in men. Br. J. Nutr. 2011, 105, 1258–1264. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.-H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.E.; Elliott, E.J. The use of low-glycaemic index diets in diabetes control. Br. J. Nutr. 2010, 104, 797–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agricultural Organisation (FAO). Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation; FAO (Food and Nutrition Paper–66); FAO: Rome, Italy, 1998; Available online: http://www.fao.org/docrep/w8079e/w8079e00.htm (accessed on 16 August 2018).
- Esfahani, A.; Wong, J.W.; Mirrahimi, A.; Villa, C.R.; Kendall, C.C. The application of the glycemic index and glycemic load in weight loss: A review of the clinical evidence. IUBMB Life 2011, 63, 7–13. [Google Scholar] [CrossRef]
- Salmerón, J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Willett, W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001, 73, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L. Dietary interventions for metabolic syndrome: Role of modifying dietary fats. Curr. Diabetes Rep. 2009, 9, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, M.; Dippenaar, N.G. Dietary fats, fatty acids and insulin resistance: Short review of a multifaceted connection. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 2005, 11, RA359–RA367. [Google Scholar]
- Kahleova, H.; Hlozkova, A.; Fleeman, R.; Fletcher, K.; Holubkov, R.; Barnard, N.D. Fat Quantity and Quality, as Part of a Low-Fat, Vegan Diet, Are Associated with Changes in Body Composition, Insulin Resistance, and Insulin Secretion. A 16-Week Randomized Controlled Trial. Nutrients 2019, 11, 615. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimzadeh Leylabadlo, H.; Sanaie, S.; Sadeghpour Heravi, F.; Ahmadian, Z.; Ghotaslou, R. From role of gut microbiota to microbial-based therapies in type 2-diabetes. Infect. Genet. Evol. 2020, 81, 104268. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, K.M.; Temple, N.J. Cereal fiber, fruit fiber, and type 2 diabetes: Explaining the paradox. J. Diabetes Its Complicat. 2018, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
| Patient/Population | Intervention | Comparator | Outcome (Primary) | Study Designs | Combining Search Terms |
|---|---|---|---|---|---|
| Patients with diabetes | Almonds | Control | Glycometabolism | Randomised controlled trial | |
| Patients with diabetes OR type 2 diabetes OR Diabetes OR Diabetes complications OR diabetes mellitus, type 2 OR diabetes mellitus | Almond OR Tree, Almond OR Almond Tree OR Sweet Almond OR Almond Trees OR Tree Nuts OR Almond, Sweet | 1. Randomised controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups 2. “Animals” NOT “Humans” 3. 1 NOT 2 | Column 1 AND Column 2 AND Column 3 |
| Citation/Country of Study | Type of Study | Sample Details and Duration of Study | Mean Age (Years) | Aim | Interventions | Results |
|---|---|---|---|---|---|---|
| Bodnaruc et al. [30] Canada | A randomised cross-over study | 7 men with type 2 diabetes. Data were collected during two experimental sessions separated by a ≥7day washout period. | 63.9 ± 2.5 | To evaluate the effects of almonds on postprandial glucose response. | Participants completed 2 experimental visits and control (white bread, butter, cheese) and test (white bread, almonds) diets were ingested. | The test meal was associated with lower postprandial glycemia and insulinemia. |
| Chen et al. [31] Taiwan | A randomised cross-over controlled study | 40 patients with type 2 diabetes. 12-weeks duration. | 54.9 ± 10.5 | To examine the effect of almonds on glycaemia | Approximately 60 g/day almonds added to a National Cholesterol Education Programme Step II diet (NCEP II) compared to NCEP II diet alone as control | Both almond-based and control diets did not significantly affect body weight and BMI or change HbA1c, fasting serum glucose, insulin, or HOMA-IR values. |
| Cohen et al. [32] USA | A randomised parallel study | 13 participants diagnosed with type 2 diabetes Almond-based diets (n = 6) Control (n = 7) 12-weeks duration. | Almond group: 66 ± 3.3 Control group: 66 ± 3.3 | To examine the impact of chronic almond ingestion on glycaemic control in patients with type 2 diabetes. | Participants were randomised to almond group (1 oz of almonds, 5 days/week) or cheese group (2 cheese sticks, 5 days/week) | HbA1c was the only blood marker that changed significantly between the treatment groups (p = 0.045). Chronic almond ingestion resulted in a 4% reduction in BMI compared with control (p = 0.047). |
| Hou et al. [6] China | A randomised controlled study | Almond group (n = 14) Peanut group (n = 11) 12-weeks duration. | Almond group: 70.86 ± 8.21 Peanut group: 68 ± 5.80 | To compare the effects of peanuts and almonds incorporated into a low-carbohydrate diet on cardiometabolic and inflammatory parameters in patients with type 2 diabetes | Peanuts or almonds were incorporated into a low-carbohydrate diet and both groups were compared after a 3-month intervention. | Almonds and peanuts have similar effect on improving fasting and postprandial blood glucose among patients with type 2 diabetes when incorporated into a low-carbohydrate diet. |
| Li et al. [33] Taiwan | Randomised cross-over clinical trial | 20 Chinese patients with type 2 diabetes. 12-weeks duration. | 58 ± 2 | To evaluate the effect of almond consumption on glycaemia in Chinese patients with type 2 diabetes | Incorporation of almonds into National Cholesterol Education Programme Step II diet (NCEP II) to replace 20% of total daily calorie intake compared with NCEP II diet alone as control. | Adding almonds into a healthy diet has beneficial effects on adiposity and glycaemic control. |
| Liu et al. [34] Taiwan | Randomised cross-over controlled feeding trial | 20 Chinese patients with type 2 diabetes. 12-weeks duration. | 58 ± 2 | To examine the effect of almond consumption on inflammation and oxidative stress in patients with type 2 diabetes | Addition of almonds (approximately 56 g/day) into National Cholesterol Education Programme Step II diet (NCEP II) to replace 20% of total daily calorie intake compared with NCEP II diet alone as control. | Adding almonds into a healthy diet could ameliorate inflammation and oxidative stress in patients with type 2 diabetes. |
| Lovejoy et al. [35] USA | Randomized double-blind crossover design | 30 participants with type 2 diabetes. 16-weeks duration. | 53.8 ± 1.9 | To assess the effects of almond-enriched diets on insulin sensitivity in patients with diabetes | The 4 diets were as follows: (1) high-fat, high-almond (HFA; 37% total fat, 10% from almonds); (2) low-fat, high-almond (LFA; 25% total fat, 10% from almonds); (3) high-fat control (HFC; 37% total fat, 10% from the MUFAs from olive or canola oil); and (4) low-fat control (LFC; 25% total fat, 10% from olive or canola oil). The almond-containing diets provided 57–113 g almonds/d depending on the total energy level | Almond-enriched diets did not influence glycaemia in patients with diabetes. |
| Ren et al. [14] China | Randomised controlled trial | 45 participants with type 2 diabetes. 12-weeks duration. | LCD group: 73.55 ± 4.99 LFD group: 70.48 ± 5.91 | To determine the effect of almond-based low-carbohydrate diet on glycometabolism, gut microbiota, and GLP-1 in patients with type 2 diabetes. | The intervention group consumed a low-carbohydrate diet, which included 56 g/day almonds that replaced 150 g/day staple food, while the control group adopted a low-fat diet education programme. | Almond-based LCD may be effective in regulating glycometabolism in patients with diabetes by stimulating the growth of SCFA-producing bacteria, increasing SCFA production and promoting GLP-1 secretion. The almond-based LCD significantly increased the SCFA-producing bacteria Roseburia, Ruminococcus, and Eubacterium. |
| Sweazea et al. [36] USA | Randomised controlled study | 21 participants with type 2 diabetes. 12-weeks duration. | Almond group: 57.8 ± 5.6 Control group: 54.7 ± 8.9 | To evaluate if almond supplementation without further dietary advice improves glycaemic control compared with control. | The almond group consumed 43 g almonds 5–7 times per week and to maintain their usual diet and activity pattern while the control group maintained their usual diet and activity pattern. | Daily almond consumption in the absence of other dietary or physical activity activities is useful in reducing inflammation in patients with type 2 diabetes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojo, O.; Wang, X.-H.; Ojo, O.O.; Adegboye, A.R.A. The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 3377. https://doi.org/10.3390/nu13103377
Ojo O, Wang X-H, Ojo OO, Adegboye ARA. The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2021; 13(10):3377. https://doi.org/10.3390/nu13103377
Chicago/Turabian StyleOjo, Omorogieva, Xiao-Hua Wang, Osarhumwese Osaretin Ojo, and Amanda Rodrigues Amorim Adegboye. 2021. "The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" Nutrients 13, no. 10: 3377. https://doi.org/10.3390/nu13103377
APA StyleOjo, O., Wang, X.-H., Ojo, O. O., & Adegboye, A. R. A. (2021). The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients, 13(10), 3377. https://doi.org/10.3390/nu13103377








